GMP Certification in Sri Lanka:
GMP Certification in Sri Lanka, Vertex Certifiers provides end-to-end GMP certification consulting services in Sri Lanka, supporting manufacturers from initial gap assessment to final regulatory and certification approval. With strong expertise in WHO GMP, Vertex works closely with pharmaceutical, food, cosmetic, and medical device facilities to build practical, inspection-ready GMP systems. Our consulting services extend across Colombo, Gampaha, Kandy, Kurunegala, Negombo, Galle, Matara, Jaffna, and Anuradhapura, delivered through flexible onsite and remote models. By combining structured documentation, hands-on training, and realistic mock inspections, Vertex Certifiers helps Sri Lankan manufacturers achieve GMP compliance efficiently, cost-effectively, and with confidence.
Good Manufacturing Practice (GMP) certification is essential for Sri Lankan manufacturers in pharmaceuticals, food, cosmetics, medical devices, and export production, ensuring consistent quality and safety amid rising global demands. As Sri Lanka boosts its $500M+ pharma exports and agro-food sectors, GMP compliance under NMRA and WHO standards unlocks international markets and regulatory trust.
What is GMP (Good Manufacturing Practice)?
GMP is a comprehensive quality system ensuring consistent production and rigorous quality control of medicinal products and related items. It safeguards product safety, efficacy, and compliance by preventing contamination, mix-ups, and errors through controlled processes. Sri Lankan facilities align with WHO GMP guidelines, PIC/S GMP for advanced pharma, and sector-specific standards, forming the backbone for local licensing and exports.
Who Needs GMP Certification in Sri Lanka?
Pharmaceutical manufacturers of APIs and formulations require GMP for product registration. Medical device producers, food and beverage processors, cosmetic and personal care makers, nutraceutical and herbal product facilities, and any export-oriented units targeting EU, US, or ASEAN markets all need certification to meet buyer and regulator mandates.
Why GMP Certification is Important in Sri Lanka
GMP fulfills licensing prerequisites, preventing market bans. It assures product safety and quality, slashing contamination risks in tropical climates. Certification grants export access, vital for Sri Lanka’s economy, while curbing recalls and penalties. Manufacturers build trust with regulators, partners, and customers, commanding premium pricing and long-term contracts.
GMP Certification Process in Sri Lanka:
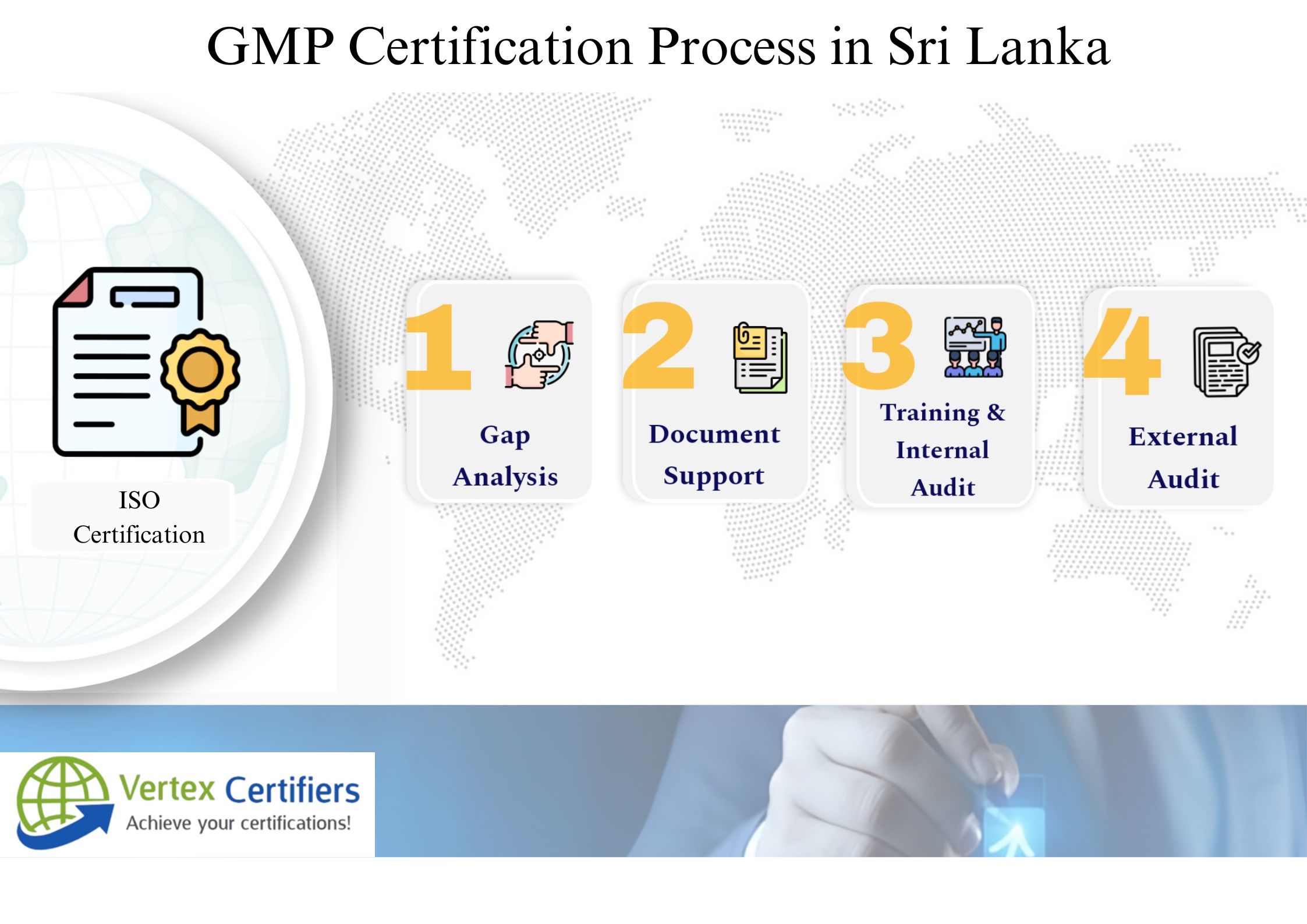
Start with GMP gap assessment against WHO’S standards, evaluating facilities, processes, and docs. Conduct facility/process review for optimal layout, hygiene, unidirectional material flow. Develop GMP documentation: Quality Manual, SOPs, batch records, validation protocols.
Implement changes with staff training across hierarchies. Run internal audits and mock NMRA inspections. Submit dossier for regulatory inspection (pre-licensing audit), resolve corrective actions, secure GMP approval, and maintain via annual surveillance.
Key GMP Requirements
- Quality Management System & GMP Policy: Senior management commitment, self-inspection, deviation handling, and change control.
- Personnel Hygiene, Training & Competency: Proper gowning, health checks, and periodic GMP training records.
- Facility Design, Utilities & Environmental Controls: Segregated areas, HVAC validation, pest control, and cleanroom classifications.
- Equipment Qualification, Calibration & Maintenance: IQ/OQ/PQ processes, scheduled calibrations, and cleaning validation.
- Material Management & Traceability: Quarantine, supplier qualification, FIFO stock rotation, and reconciliation.
- Production & Process Controls: Batch manufacturing records, in-process checks, and yield calculations.
- Quality Control & Testing: Independent QC lab, OOS investigations, and stability studies.
- Documentation, Records & Data Integrity: Controlled forms following ALCOA+ principles, retention for 5+ years.
- Complaint Handling & Product Recall Systems: SOPs for vigilance and annual mock recalls.
Timeline for GMP Certification in Sri Lanka
Small to mid-size facilities generally achieve GMP certification in 1–3 months, including 1 month for assessment and documentation, 1–2 months for upgrades and staff training, followed by inspection. Large or complex sites take 3–6 months, especially if sterile validations or multi-line setups are involved. Timelines depend on readiness (greenfield vs retrofit), product risk, and inspection scheduling.
| Facility Type | Timeline | Potential Delays |
|---|---|---|
| Small / Mid-size | 3–6 months | Documentation preparation |
| Large / Complex | 6–9 months | Validations, HVAC, utilities |
| Sterile / High-risk | 9–12 months | Utility qualifications & re-audits |
Common GMP Challenges for Sri Lankan Manufacturers
Aging facilities in Colombo or export zones often require costly layout or HVAC upgrades to meet airflow standards. Shop-floor staff frequently lack GMP awareness due to artisanal traditions. Documentation gaps arise from manual or inconsistent records. Utility unreliability—including humid water systems and intermittent power—can threaten compliance. Meeting export standards like EU GMP can exceed local norms, challenging SMEs.
Why Choose Vertex Certifiers for GMP Certification in Sri Lanka?
Vertex Certifiers brings WHO & PIC/S GMP expertise, delivering end-to-end guidance from gap assessment to regulatory approval. Services include practical SOPs, hands-on staff training, and mock audits simulating real inspections. Our hybrid remote and onsite consulting covers Sri Lanka coast-to-coast—from Kandy factories to Galle seafood processors. Our cost-effective, phased approach accelerates certification while maximizing ROI for export-focused manufacturers.
Conclusion
GMP certification strengthens Sri Lankan manufacturers’ product quality, regulatory compliance, and export potential, transforming compliance into a strategic gateway for sustainable global growth.
Planning GMP Certification in Sri Lanka?
Contact Vertex Certifiers for a free GMP gap assessment and a customized GMP implementation roadmap.
📧 Email: info@vertexcertifiers.com
Get Started TodayOur Services
- GMP Certification
- GLP Certification
- GDP Certification
- Halal Certificate
- Organic Certificate
- CE Marking Certification
- RoHS Certification
- FDA Certification
- CMMI Certification
- Cyber Security
- VAPT Testing
- Security Assessment
